Nov 10. 2023
Synthetic Method Of Magnesium Sulphate
Synthetic Method Of Magnesium Sulphate
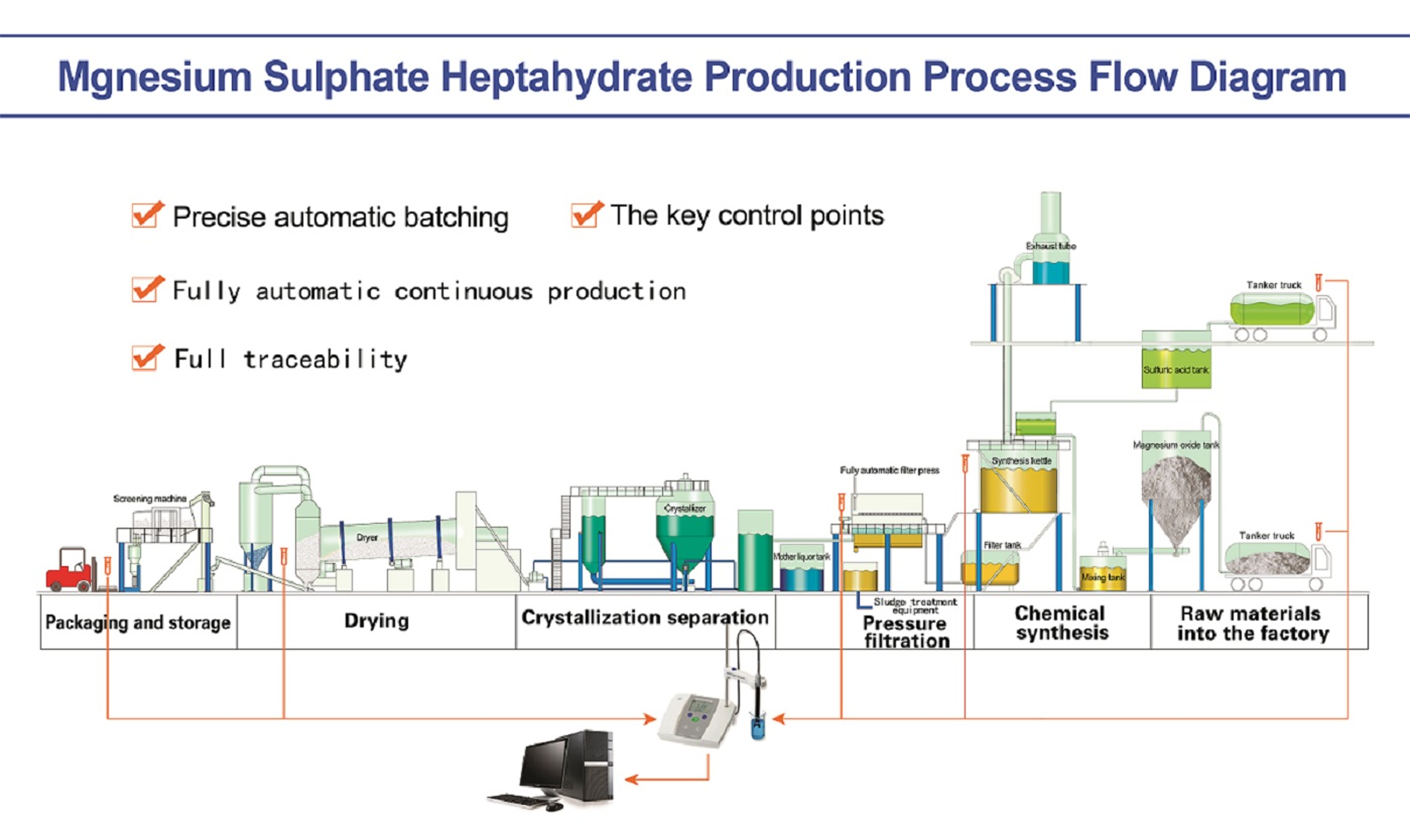
Use Sulfuric Acid: Sulfuric acid and magnesium oxide , magnesium hydroxide or
magnesium carbonate through reaction,filtration, insulation, crystallization, separation,
drying derived . The reaction equation as follows:
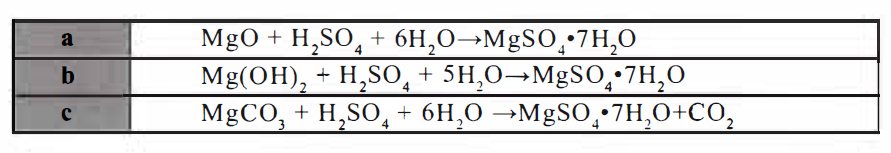
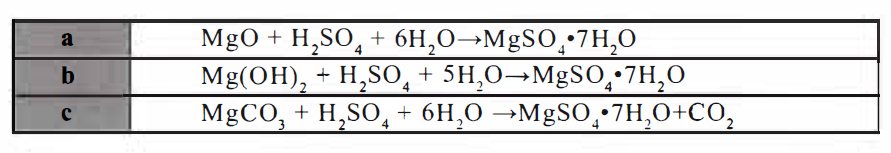
- Used Industrial recovery of sulfuric acid to produce magnesium sulfate
- High temperature salt system manufacture_magnesium sulfate and sodium chloride
- Szaibelyite and sulfuric acid preparation of boric acid will produce byproduct,the byproduce is magnesium sulfate
- Bittern preparation of magnesium sulfate in sea salt production
- Use the "three highs method preparation of magnesium sulfate , sodium chloride and potassium chloride
- A byproduct in the preparation of glazed tile to produce magnesium sulfate
- Obtained magnesium sulfate in the boric acid mother liquor


- The first ,second and third technical technical take up most percentage of domestic market:
- The first one take priority of high purity,low impurity and appropriate for top market.
- The second one will supply low purity product with more impurity,however the cost is low and use in the lower end of market.
- The third one is natural product with low cost and high Fluorine and mineral element,mainly used in the field of agriculture, and limited in the field of food pharmaceutial and industry.